During the last few years, the number of precision medicine drugs approved by the FDA has increased steadily, far outpacing the growth of one-size-fits-all, non–precision medicine treatments. Intrinsic to this trend is the growing dependency on precision testing to select the most appropriate patient population for these precision medicine drugs.
To better understand the level of dependency that these precision medicine therapies have on an efficient diagnostic testing ecosystem, Diaceutics expanded its definition of precision testing in 2018 to achieve more real-world data insights.1,a This real-world definition includes three different types of tests upon which key precision medicine treatments are dependent: companion, complementary and conduit diagnostic tests. Within the precision medicine armamentarium, these tests are either required (i.e., companion diagnostics) or suggested (i.e., complementary and conduit diagnostics) for these treatments.2,3
Specifically, companion diagnostics are cited in the therapy label as required to guide therapy treatments (in the selection of appropriate patients).2 Complementary tests,b on the other hand, are non-requisite yet frequently used tests cited in the therapy label to support therapy treatments (in more accurately identifying appropriate patients).3 Complementary tests help predict patient response to precision medicines by identifying “a biomarker-defined subset of patients that respond particularly well to a drug and aid risk/benefit assessments for individual patients, but that are not pre-requisites for receiving the drug.”4 Lastly, conduit tests are screening, diagnostic, or monitoring tests that help direct patients to the “right drug or therapeutic dose.”1 They may or may not appear in the therapy label but are instrumental to placing and/or managing patients on therapy.
Using this expanded real-world definition of precision testing, Diaceutics’ data reveal a significant number of therapies that have a high dependency on efficient diagnostic pathways for patients. Our definition has, therefore, extended beyond companion diagnostics to fully articulate the real-word expanse of precision testing.
In 2019, the FDA issued 78 drug approvals.5 Some approvals were for novel drugs (N=45) and others were for extended use of existing therapies (N=33), in which previously approved drugs received additional indications. Our analysis of FDA approvals of precision medicine drugs in 2019 suggests the following conclusions5:
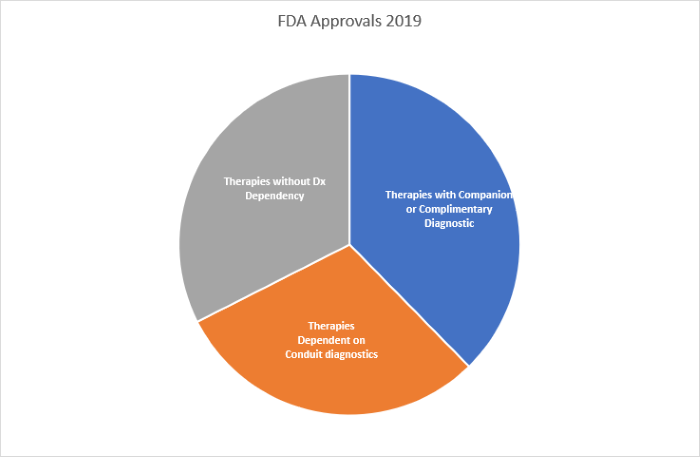
- The majority of all FDA approvals (68% [53/78]) were of therapies with a direct or high dependency on efficient patient diagnostic testing (See Figure 1).
- Of all approvals in 2019,
- 37% (29/78) were for therapies with companion or complementary biomarkers cited in the drug label (See Figure 2).
- 29% (23/78) were for therapies with a dependency on conduit diagnostics instrumental to placing and or managing the patient on therapy (See Figure 2).
- Only 32% (25/78) were for therapies with low or no dependency on efficient patient testing (See Figure 2).
- Of the 45 novel therapies approved,
- 58% (26/45) were for therapies with a high or direct dependency on efficient patient testing.
- 27% (12/45) were for therapies with companion or complementary biomarkers cited in the drug label.
- 29% (13/45) were for therapies with a dependency on conduit diagnostics instrumental to placing and or managing the patient on therapy.
- 42% (19/45) were for therapies with low or no dependency on efficient patient testing.
- Of the 33 therapies granted extended indication approvals,
- 82% (27/33) were for therapies with a high or direct dependency on efficient patient testing/likely to be enabled by tests in the market. Many of these drugs were approved via rapid review, which further supports the business case for precision therapy, suggesting that Pharma is finding it easier to gain approvals for extended uses with biomarker enabled therapies.
- 52% (17/33) were for therapies with companion or complementary biomarkers cited in the drug label.
- 30% (10/33) were for therapies with a dependency on conduit diagnostics instrumental to placing and or managing the patient on therapy.
- Only 18% (6/33) were for therapies with low or no dependency on efficient patient testing.
- PREDICTION: Based on our analysis of 517 phase 3 clinical trials of test-dependent therapies, presented in our Pharma Precision Medicine Report 2019,6 Diaceutics expects that the number of approved precision medicine assets with a biomarker cited in the therapy label as essential or recommended for prescribing will exceed non-precision medicine assets in the years 2020 and 2021. In line with current trends, Diaceutics believes that most of the approved drugs will be cancer treatments.
aAccess the Diaceutics 2018 Pharma Precision Medicine Readiness Report podcast here: https://www.diaceutics.com/?webinar=podcast-pm-readiness-report-2018.
bThe FDA has not yet officially defined complementary diagnostics but, instead, has provided this draft definition.4
References
- PM Readiness Report 2018. Podcast. https://www.diaceutics.com/?webinar=podcast-pm-readiness-report-2018. Published April 20, 2018. Accessed March 3, 2020.
- Companion diagnostics. FDA website. https://www.fda.gov/medical-devices/vitro-diagnostics/companion-diagnostics. Published December 7, 2018. Accessed February 28, 2020.
- Jørgensen JT. Companion and Complementary Diagnostics: Clinical and Regulatory Perspectives. Trends Cancer. 2016 Dec;2(12):706-712. doi: 10.1016/j.trecan.2016.10.013.
- Theoret M. Biomarkers for PD-1/L1 inhibitors: Regulatory considerations. Presented at the EMA-CDDF Joint Meeting; February 4-5, 2016; London. https://www.ema.europa.eu/en/documents/presentation/presentation-biomarkers-pd-1/l1-inhibitors-regulatory-considerations-marc-theoret-s31_en.pdf. Accessed February 27, 2020.
- Novel drug approvals for 2019. FDA website. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019. Published January 14, 2020. Accessed February 28, 2019.
- Pharma Precision Medicine Readiness Report 2019. Diaceutics website. https://www.diaceutics.com/pmreport19/. Published October 2019. Accessed February 27, 2019.
Figure 1. Analysis of all FDA therapy approvals 2019 by Dx dependency.
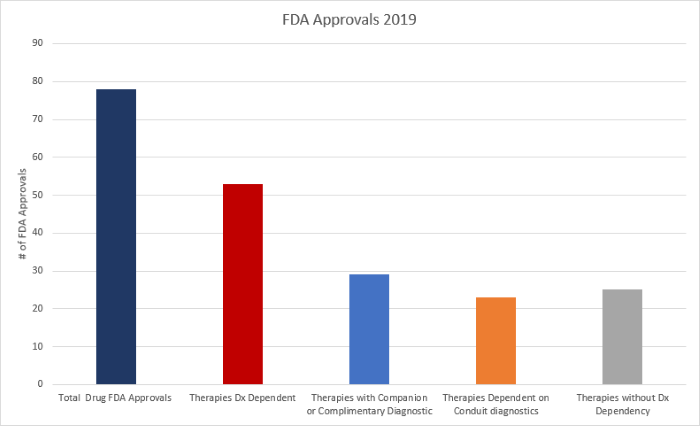
Figure 2. Analysis of all FDA therapy approvals 2019 by % share.