“When you hear hooves, think horse, not zebra.” This is a common principle new physicians are taught to highlight that the odds are their patient has a common diagnosis rather than a rare one. However, with advancement in technologies and sequencing, we now have a better understanding of the genetic origin of diseases and that each patient has a different molecular profile/set of alterations that drives their individual disease. So, is this principle still the case?
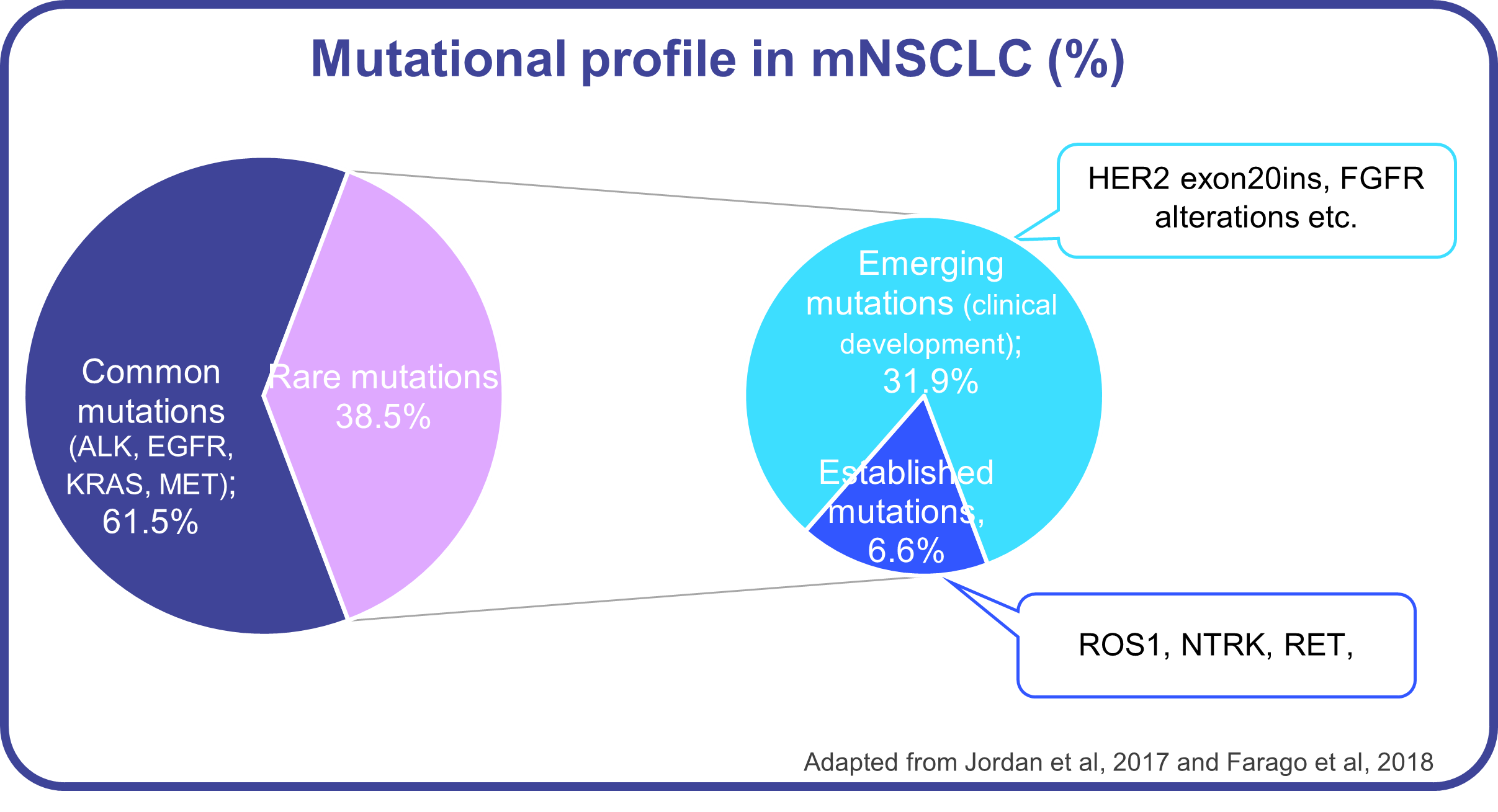
Let’s take NSCLC as an example. Mutations in EGFR and KRAS constitute around half of the cases; alterations in ALK and MET add another 10%. However, beyond these biomarkers there are 40% of patients whose tumors are driven by mutations in less common biomarkers, (i.e., individually have < 3% prevalence), such as ROS1, ERBB2 exon20ins, BRAF V600E, RET, NTRK, FGFR, PIK3CA or BRCA1/2. Some of these biomarkers have recently approved targeted therapies, while others are still in clinical development. Yet, while around 70-80% of NSCLC patients are tested for EGFR, ALK or ROS1, less than 50% are evaluated for BRAF, NTRK or other rare established or emerging biomarkers, (especially in the community setting where 80% of patients are treated).
One reason for this is the fact that almost a third of NSCLC testing is still performed by single gene technologies (e.g., PCR, IHC) instead of a comprehensive NGS panel, driving oncologists to prioritize which biomarkers to test. Even when an NGS panel is requested, coverage for all rare biomarkers might be suboptimal. We recently showed that only 62% of the top 25 labs in US offered a comprehensive panel that can detect all common and rare mutations. This eventually prevents many patients from receiving the most effective, personalized medicine. So, let’s not forget that NSCLC, or even a more precise subtype such as lung adenocarcinoma, is not one disease, but a set of diseases, each driven by different biomarkers with different therapeutical approaches.
So how can we change this to ensure all patients are receiving timely and accurate diagnosis as well as the most appropriate therapies, regardless of how common or rare their mutations or indications are? It will start by improving all stakeholder awareness and education on firstly, rare diseases in general; secondly, on specific rare biomarkers through unbranded campaigns; and thirdly, on genetics and the use and importance of prioritizing comprehensive genomic testing. International collaborations will foster these efforts and will enable broad real-world data collection for improving preclinical and clinical research. A change in trial design and regulatory requirement are also required, but these will be covered in another blog.
We at Diaceutics can support by improving access and adoption of testing for rare biomarkers or indications by better understanding the Dx market, its barriers and opportunities to building and implementing a Dx strategy to improve lab readiness and physician awareness/adoption. In addition, DXRX Signal provides weekly lists of physicians who had a biomarker positive patient in the previous week to accelerate identification of patients for treatment or clinical trial enrollment and reduce any patient leakage.
Let us end by emphasizing that we are in an era where rare mutations are increasingly emerging as important targets for research, accurate diagnosis and treatment decisions. So, for some diseases, when we hear hooves, let’s not forget or overlook the hidden zebra. After all, if you don’t suspect it, you can’t detect it!
Diaceutics Data Repository
Diaceutics IASLC poster 2021: https://www.jto.org/article/S1556-0864(21)03053-7/fulltext